Side effect of new Alzheimer’s drugs: Brains shrink massively
New study shows antibody drugs cause even more side effects
In recent months, the Alzheimer’s drug “Lecanemab” (brand name: “Leqembi”), approved in the United States under emergency FDA approval, has generated considerable debate. Although the antibody is said to reduce amyloid load – that is, the protein deposits between nerve cells – by 27% in mild Alzheimer’s dementia, the side effects for patients were already critically evaluated late last year in terms of potential benefits.
That’s because it turned out that among the 900 “verum patient:ing” subjects actually treated with lecanemab, 12.5% suffered brain swelling and 17% suffered cerebral hemorrhages – both of which can be life-threatening. Various deaths have also been reported, although the extent to which these deaths are directly related to the active substance has not been clarified to date.
Nevertheless, lecanemab is still being treated as a “beacon of hope” and a “milestone”. But now there are further concerns: substances such as this antibody, which act against amyloid deposits in the brain, at the same time also cause the brain to shrink further.
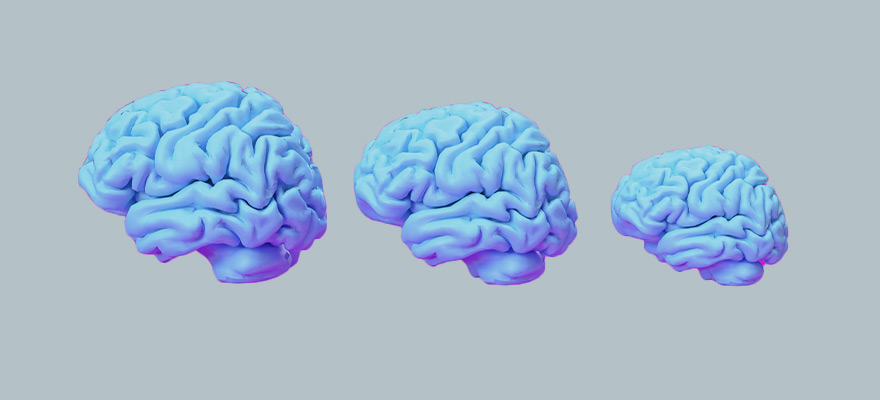
New meta-study reveals: Amyloid antibodies shrink the brain by more than a quarter
In late March 2023, a meta-study was published in the prestigious journal NEUROSCIENCE, presenting a comprehensive study analysis. The study shows that beta-amyloid and amyloid plaque medications are associated with further brain shrinkage in Alzheimer’s disease patients.
The analysis included 31 studies of adult participant:s and used MRI measurements to track changes in brain volume. The results showed that treatment with these drugs was associated with a decrease in brain volume. Some drugs, called secretase inhibitors, led to accelerated atrophy of the brain.
In two large trials of lecanemab, those participants:in who received the highest dose of the drug lost an average of 28% more brain volume than those who received a placebo. Although a spokesperson for Eisai, the pharmaceutical company developing the drug, argued that this shrinkage could be a “good” sign, since the antibody removes amyloid deposits and reduces inflammation. However, other scientists expressed concern, emphasizing that volume loss on MRI is generally considered unfavorable.
Overall, Alzheimer’s drug research remains complex and fraught with many uncertainties. While on the one hand, some promising results are being obtained, on the other hand, there are increasing concerns about possible short-, medium-, and long-term adverse effects of lecanemab and similar compounds on patient health and well-being.
Brain stimulation procedure TPS on the rise: no such side effects detectable
Neurostimulation procedures such as Transcranial Pulse Stimulation (TPS) have not been found to have side effects of this type in a single case to date. On the contrary, in studies conducted at the University of Vienna, researchers have already been able to prove that TPS counteracts cortical atrophy, i.e. the loss of brain substance.
As of April 01, 2023, Transcranial Pulse Stimulation is now available in more than 160 clinics, university hospitals and applying practices in over 30 countries – and the trend is rising. This is also due to the fact that the study situation is becoming more and more comprehensive, more and more new mechanisms of action are being discovered, deciphered and investigated, and finally an important advantage is becoming more and more apparent: With TPS, only marginal side effects occur, if at all in individual cases (2 out of 100), such as mild headache or dizziness, which quickly pass on their own within a very short time without the need for medication.
Source:
https://n.neurology.org/content/early/2023/03/24/WNL.0000000000207156